Nuclear power is widely recognized as a cleaner energy source compared to fossil fuels, as it avoids the emission of air pollutants and greenhouse gases like carbon dioxide. However, it does produce radiotoxic waste that requires careful treatment to avoid negative environmental and health impacts.
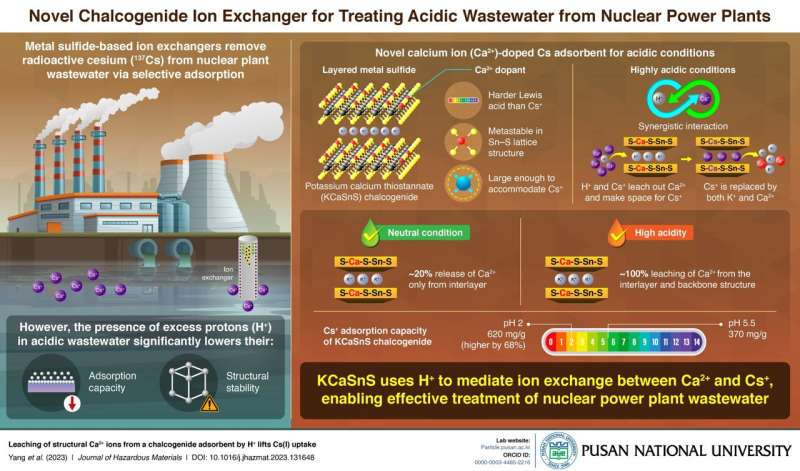
One of the main by-products of the nuclear fission process used for power generation is radioactive cesium-137 (^137Cs). This radioactive element, which has a half-life of 30 years, is typically removed from nuclear powerplant wastewater through selective adsorption using ion exchangers. However, in acidic wastewater, the excess protons (H+) present hinder the adsorption process and damage the adsorbent’s lattice structure.
A research team led by Prof. Kuk Cho from Pusan National University in Korea has recently discovered a way to turn this challenge into an opportunity. Their groundbreaking work, published in the Journal of Hazardous Materials, introduces a new calcium-doped ion exchanger called potassium calcium thiostannate (KCaSnS).
This novel adsorbent utilizes the problematic H+ ions in acidic wastewater to enhance the adsorption of cesium ions. The Ca2+ ions from KCaSnS are leached out by H+ and Cs+, creating space for Cs+ ions to be adsorbed.
Prof. Cho explains, “By incorporating Ca2+ into the Sn–S matrix, we transformed the troublesome protons into functional agents, resulting in a metastable structure. Additionally, Ca2+ is a harder Lewis acid than Cs+ and can easily leave the lattice due to its weaker affinity to the Lewis soft base S2- under acidic conditions. This creates enough space for Cs+ ions to reside after they are released from the lattice structure.”
In their study, the team used a hydrothermal process to synthesize the KCaSnS ion-exchange material. They then investigated its adsorption capacity for non-radioactive Cs+ ions in solutions with pH values ranging from 1 to 13, ensuring safety from radioactivity exposure.
The team found that at neutral pH 5.5, the Cs+ adsorption capacity was 370 mg/g, while at strongly acidic pH 2, the capacity increased by 68% to 620 mg/g. Interestingly, this trend contradicted previous studies.
This observation can be attributed to the fact that under neutral conditions, only around 20% of the total spots available for Cs+ adsorption were accessible, with Ca2+ ions leaching out solely from the interlayers. In contrast, under highly acidic conditions, nearly 100% of Ca2+ ions were leached out from both the interlayer and the backbone structure, providing more space for Cs+ ions within the lattice. Additionally, interlayer K+ ions were involved in the ion exchange process in all cases.
These results highlight KCaSnS as a highly promising solution for removing radioactive ions from nuclear powerplant wastewater. The insights gained from this study have the potential to drive the development of high-performance adsorbents for highly acidic environments. Prof. Cho suggests, “The impressive adsorption capacity of KCaSnS can help address the challenges associated with managing radioactive waste by reducing the volume of waste produced during spent fuel reprocessing and decommissioning of nuclear power plants.”
More information:
Chenyang Yang et al, Leaching of structural Ca2+ ions from a chalcogenide adsorbent by H+ lifts Cs(I) uptake, Journal of Hazardous Materials (2023). DOI: 10.1016/j.jhazmat.2023.131648
Provided by Pusan National University
Citation:
A new adsorbent for removing radioactive cesium ions from nuclear wastewater (2023, June 20)
retrieved 20 June 2023
from https://phys.org/news/2023-06-adsorbent-radioactive-cesium-ions-nuclear.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without written permission. The content is provided for information purposes only.



